 By Curtis Walcker, M.S.
By Curtis Walcker, M.S.
January 24, 2021
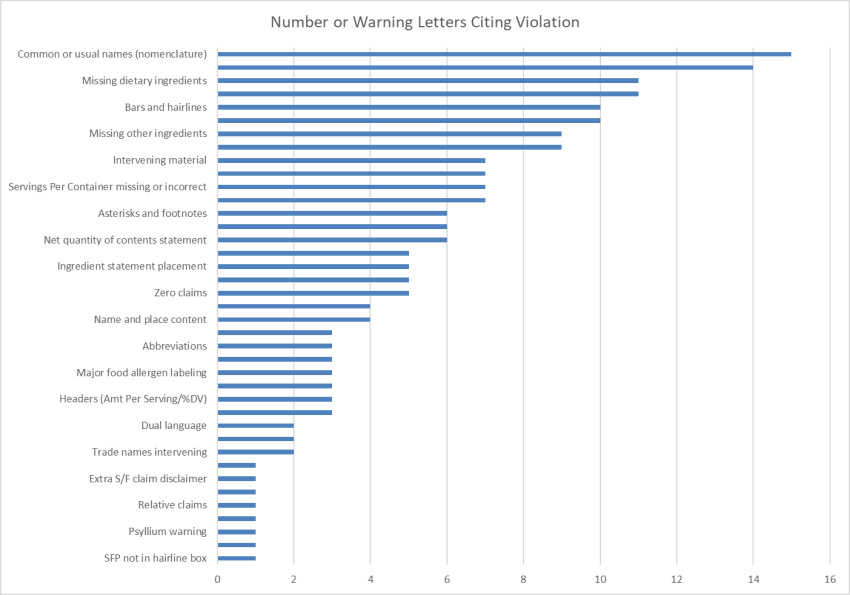
In 2020, we saw 20 FDA Warning Letters posted citing label violations for dietary supplements. We tallied up all of the violations cited and put them into a single chart. For the most part, these are the same violations we see year after year. However, there were a few things worth taking note of. Here is what we found:
What was familiar?
We do tracking and compiling of FDA Warning Letters each year, and with regard to label violations, everything was pretty familiar. FDA was able to grab the same old low-hanging fruits that brands still find challenges with. As with years previous – incorrect common or usual names, missing plant parts for botanicals, missing ingredient declarations, and incorrect use of bars and hairlines topped the chart with very high numbers. Things like zero claims, intervening material, and the use of footnotes that should not be present can typically be traced back to brands trusting the templated system-generated Supplement Facts panels supplied to them by their manufacturers, or worse – copying competitor labels. No matter what the reason behind errors, the Warning Letters get addressed to the brand on the bottle, and not manufacturers or competitors. Shoring up label compliance is definitely one of the fastest and easiest risk-reducers for any brand.
How did our client labels compare?
New client labels were very similar in terms of what issues we resolved, and frequency in which they occurred. The great thing that occurs with returning or existing clients is that within a few labels together, the client begins removing template errors across all of their labels proactively, so our reviews become more streamlined and focused on product-specific errors. For instance, once a client figures out that they were missing serious adverse event reporting information on a couple of labels, they go back and put it onto all labels.
What stuck out?
More than anything, this is why we track Warning Letters and violations the way we do. You can go read the laws and regulations all day long, but Warning Letters shed light on what the FDA is focusing on, and how they are interpreting things. While nothing was really new here, beyond the usual violations, these three stuck out:
- Trade names are not permitted within the Supplement Facts panel.
This may come as a big surprise to virtually all brands…and manufactures…and raw material suppliers. However, it is the case. The information panel of a label generally bears the Supplement Facts panel, ingredient statement, and name/place of business, and major food allergen labeling when applicable. All of these items are regulated in terms of their contents, placement, and formatting. Adding anything beyond what is required, becomes at risk of being what the regulations refer to as “intervening material”. And anything intervening and not required by the regulations is generally not allowed.
In the Warning Letter to Market America, Inc. for instance, FDA noted to them that referencing their trademarked ingredients (SunActive® and Quatrefolic®) within the Supplement Facts panel was not permitted – referring to it as intervening material.
This does create a bit of a conundrum. A brand invests more into their product with trademarked ingredients, and they want to let their consumers know. However, for FDA compliance, the Supplement Facts panel should not be that marketing vehicle. There are many other places more suitable on the label or in the labeling for marketing callouts.
- “Herb” and “aerial parts” are not acceptable plant parts to declare for botanicals.
Again, probably surprising to a lot of brands. We see this come through on labels weekly. Plant part(s) declared for botanicals need to be specific. Understandably, FDA told R-Garden, LLC that “Cleavers herb” was inadequate, as “herb” is not a plant part. Less understandable, however, ForYou, Inc. and BHP Holdings, Inc. were told that “aerial parts” was also inadequate. The industry tends to use “aerial parts” when plant parts used consist of all parts above ground – much like “whole plant” is used when all parts are used. The challenge in labeling aerial parts one-by-one is that the lists could become long and ultimately be more misleading when parts are left out of the list. This is one we’d like to see some more clarity from the FDA on what exactly they are looking for.
- If a footnote in the Supplement Facts panel is not required, it is not allowed.
This one is usually just a template error, usually passed along by a manufacturer, but a risk nonetheless. We see many Supplement Facts panels with both of the typical footnotes, “Daily Value not established” and “Percent Daily Values are based on a 2,000 calorie diet”. However, either one is only required and only allowed when it applies based on what is declared. FDA let LifeHealth Science know this, when they sent them a Warning Letter stating that their footnote was not permitted, as nothing declared required it.
If you recognize any of these errors in your own labels, or are unsure, we are here to help. We offer very affordable label compliance review service, fast turnaround times, and bulk discounting for multiple labels. Contact us today!
