By Curtis Walcker, M.S.
October 25, 2015 (Updated October 29, 2017)
 This month’s article highlights five mistakes in label compliance that we commonly see in our dietary supplement label reviews.
This month’s article highlights five mistakes in label compliance that we commonly see in our dietary supplement label reviews.
1. Copying label design from other dietary supplement labels
A tell-tale sign of label design plagiarism is when the label appears to be a hybrid of compliance and non-compliance. Sometimes this is in the form of a nutrition label that says “Supplement Facts” across the top, but then has the design elements for conventional foods. Other times, some label components are very compliant, while others indicate a clear lack of knowledge of the labeling regulations. No matter what the issues are, the bottom line is that copying someone else’s label to create your own is a very bad practice. You never know how well the other company understood the regulations, or to what level they complied with them. Your interpretation of their label as it applies to yours will probably even create further problems.
2. Continuing to make errors just because you always have
When your labels are up for reprinting, it is a great time to send them through the review process with someone knowledgeable about the regulations. If changes must be made for better compliance, do not resist. Just because errors have been made for long periods of time without detection, it does not mean that they are any more correct. If a claim is being made that should not be made, or a Serving Size has been wrongly declared, correct it as soon as possible. Marketing messages and formulations can be adjusted with some creativity. The labeling regulations are far less flexible.
3. Assuming that certain label violations are low risk
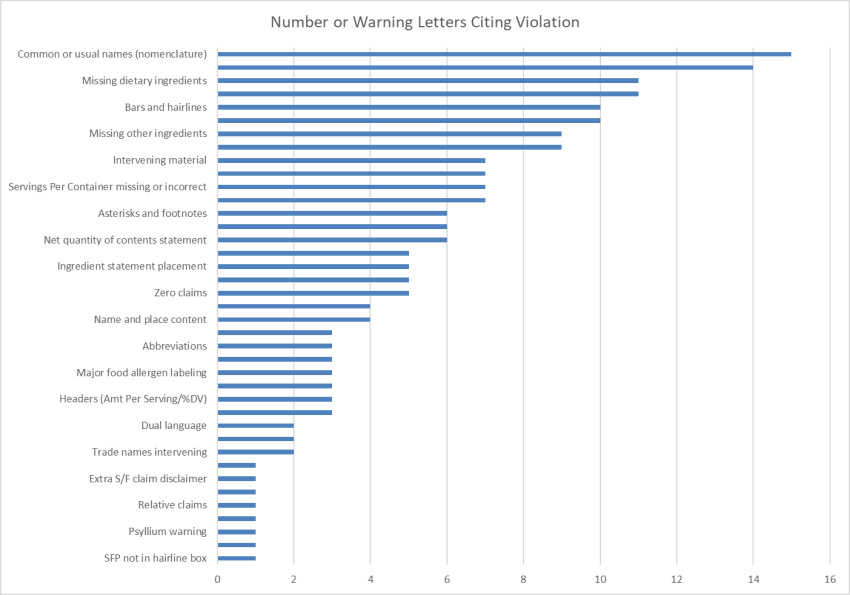
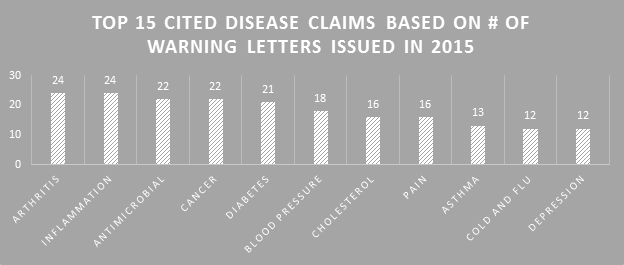
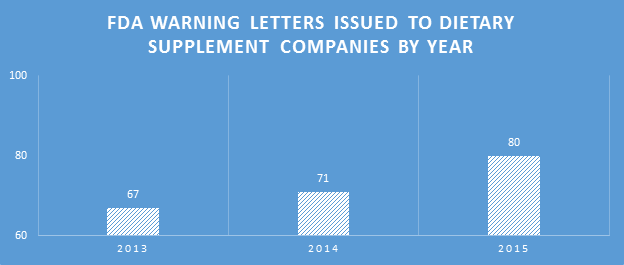
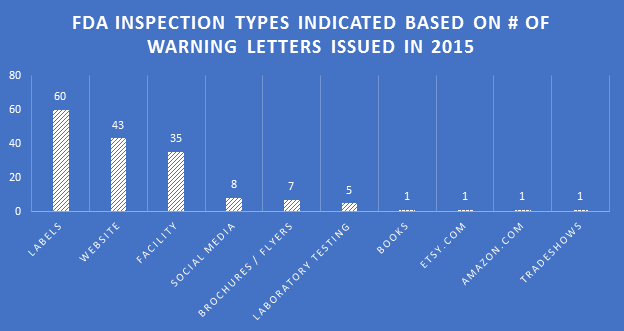
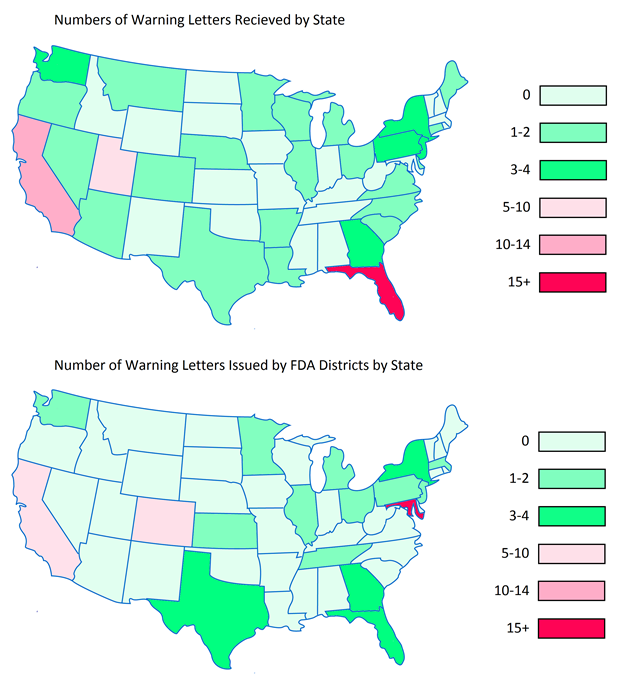
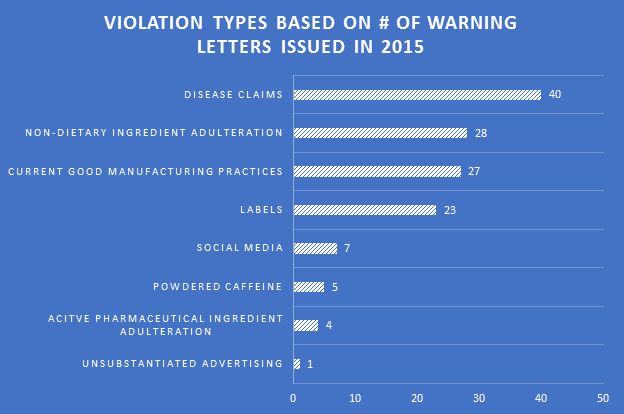
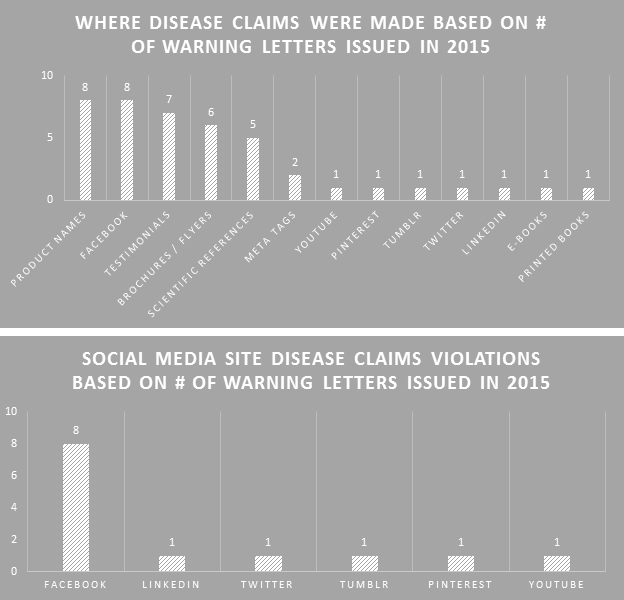
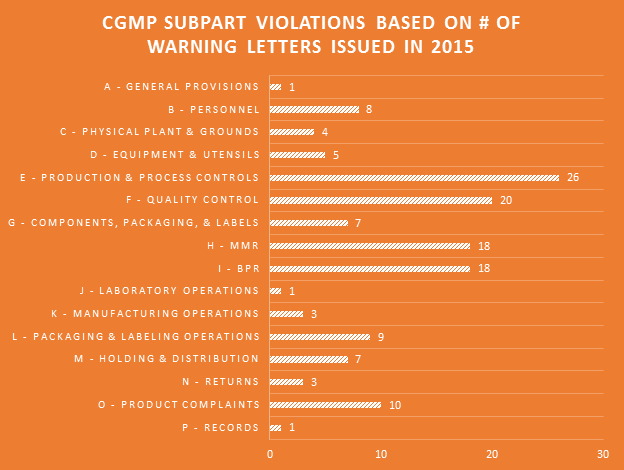
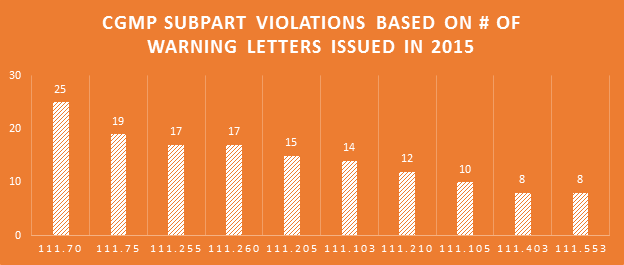
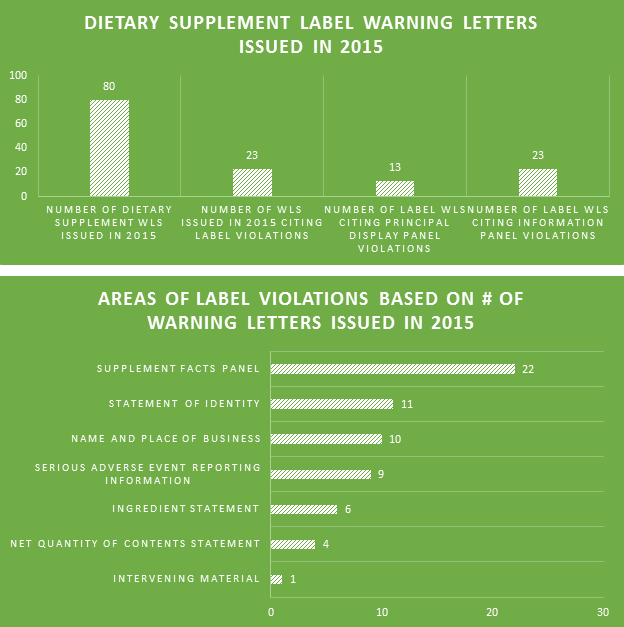
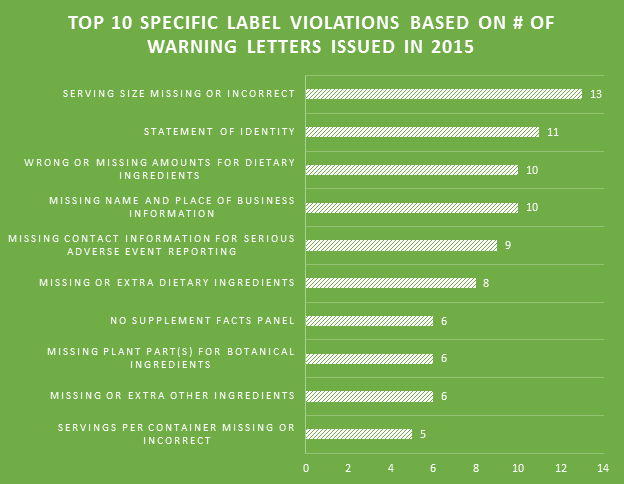
Making the assumption that certain aspects of label compliance are lower risk or lower priority than others can be a huge mistake. Many times in our label reviews, clients might not see the purpose of our requested changes, or opt not to make them with their own assessment that the requests are low risk. Our explanation is first the regulations that require the change, and second the fact that the FDA’s label inspections have been increasingly technical over the years, as evidenced by Warning Letters citing seemingly minor violations. Having a Warning Letter come up on the internet when people search for your company is not worth the risk of maintaining label problems for no good reason. Not to mention the growing number of clients we are getting that have their products detained at the borders because of very minor violations.
4. Using the wrong units of measure
This we see a lot. Sometimes it is because the company was not aware of the proper units of measure to use for declaring ingredient amounts; other times it is because they wanted things to appear better on their label. In most cases, when our review finds that something declared in mg, for example, needs to be declares in g, we are met with some reluctance. The company usually does not want to go from declaring 1,500 mg of an ingredient to 1.5 g, even though that is what the regulations might require. Like the mistake above, maintaining something like this might seem minor and low risk, but could find its way into a Warning Letter or cause a shipment to be detained at a border.
Update: The old label regulations read, “These amounts shall be expressed using metric measures in appropriate units (i.e., 1,000 or more units shall be declared in the next higher set of units, e.g., 1,000 mg shall be declared as 1.1 g).” The new label regulations remove the example language, and now only reads, “These amounts shall be expressed using metric measures in appropriate units.” Time will tell how the enforcement will change.
5. Making “zero claims”
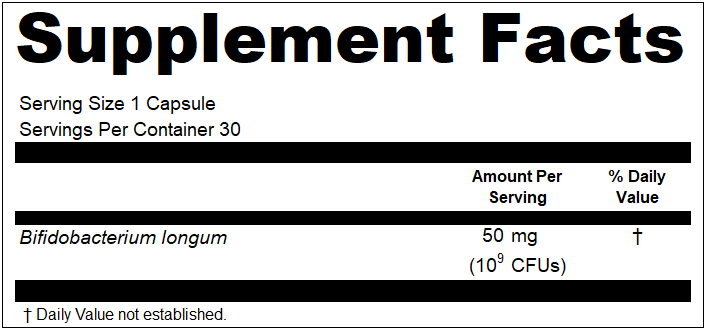
“Zero claims” are essentially claims made in the Supplement Facts panel that show an ingredient is present at a value of zero (e.g., Total Fat 0 g). These find their way onto labels for many reasons. Sometimes it is label plagiarism, many times it is marketing, and other times companies feel it is required. On conventional food labels, there are requirements for making zero claims. However, on dietary supplement labels, you are not allowed. If you have zeros under your Amount Per Serving heading, they must be removed.
Hopefully none of these frightening mistakes are ones that you are currently making. If you are, it would be worth reviewing them at your next label printings or sooner. Keep in mind that these are just a slim fraction of what we see on a regular basis. If you have questions or need full label review, please contact us anytime.




 If you are a frequent reviewer of dietary supplement labels, you have probably found that your speed and efficiency increase when you have your “tools” readily accessible. All the way back in 2014, we published the
If you are a frequent reviewer of dietary supplement labels, you have probably found that your speed and efficiency increase when you have your “tools” readily accessible. All the way back in 2014, we published the 
 By Curtis Walcker, M.S.
By Curtis Walcker, M.S.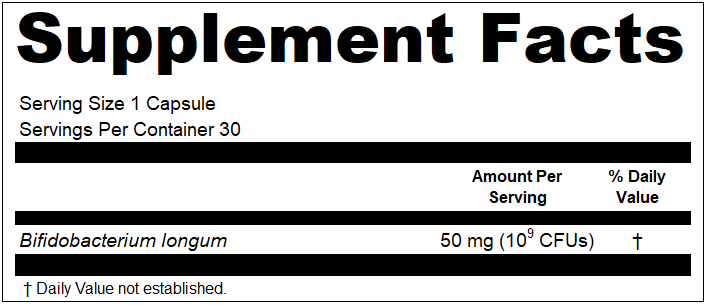



 This month’s article highlights five mistakes in label compliance that we commonly see in our dietary supplement label reviews.
This month’s article highlights five mistakes in label compliance that we commonly see in our dietary supplement label reviews.
 If you are a frequent reviewer of dietary supplement labels, you have probably found that your speed and efficiency increase when you have your “tools” readily accessible. This month we have prepared a two-part series focusing on a selection of the important tools of our trade. If you are not currently using some of these, we help direct you to where you may find them.
If you are a frequent reviewer of dietary supplement labels, you have probably found that your speed and efficiency increase when you have your “tools” readily accessible. This month we have prepared a two-part series focusing on a selection of the important tools of our trade. If you are not currently using some of these, we help direct you to where you may find them.