By Curtis Walcker, M.S.
November 8, 2015
 Many professions require continuing education to keep the professionals in those fields abreast of the trends and evolving research. For us that are involved in dietary supplement regulatory affairs, one of the best unofficial methods of continuing education comes in the form of FDA Warning Letters. A careful reading of these each week not only reminds us of the regulations, but shows us how the FDA is interpreting them, and which issues might be on their radar. In addition, when we are suggesting that our companies or client’s make changes to their manufacturing practices, labels, claims, etc., it can be easier at times to get buy-in when not only the regulations, but also one or more recent FDA Warning Letters can be brought to the discussion table to demonstrate the needs and the associated risks.
Many professions require continuing education to keep the professionals in those fields abreast of the trends and evolving research. For us that are involved in dietary supplement regulatory affairs, one of the best unofficial methods of continuing education comes in the form of FDA Warning Letters. A careful reading of these each week not only reminds us of the regulations, but shows us how the FDA is interpreting them, and which issues might be on their radar. In addition, when we are suggesting that our companies or client’s make changes to their manufacturing practices, labels, claims, etc., it can be easier at times to get buy-in when not only the regulations, but also one or more recent FDA Warning Letters can be brought to the discussion table to demonstrate the needs and the associated risks.
This month’s article is a short story about a recent FDA Warning Letter to a Company called New Dawn Nutrition, Inc., (previously) dated 8/7/2015. An excerpt of one of the cited violations for their labels read as follows:
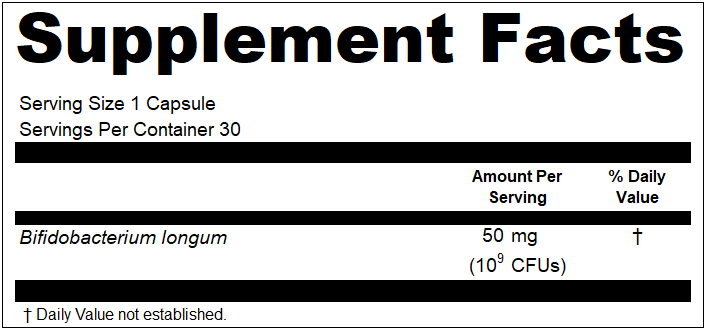
“Your “NDNFA Prime Pure Super Protein” (Cake Batter and Vanilla Milkshake varieties) product labels declare potassium present at “5%” of the Daily Value, phosphorus present at “9%” of the Daily Value, and calcium present at “17%” of the Daily Value~ however, according to 21 CFR 101.9(c)(8)(iii), the percentages for vitamins and minerals shall be declared in increments of 2-percent up to and including the 10-percent level and the nearest 5-percent increment above 10-percent and up to and including the 50-percent level.”
This violation would have been pretty mundane if it was cited for conventional food products, but these were dietary supplements. And for dietary supplements, there is a separate regulation from 21 CFR 101.9(c)(8)(ii), which is 21 CFR 101.36(b)(2)(iii)(C), that reads:
“The percentages based on RDI’s and on DRV’s shall be expressed to the nearest whole percent, except that for dietary ingredients for which DRV’s have been established, “Less than 1%” or “<1%” shall be used to declare the “% Daily Value” when the quantitative amount of the dietary ingredient by weight is great enough to require that the dietary ingredient be listed, but the amount is so small that the “% Daily Value” when rounded to the nearest percent is zero (e.g., a product that contains 1 gram of total carbohydrate would list the percent Daily Value as “Less than 1%” or “<1%”).”
Further, the Dietary Supplement Labeling Guide provides the following:
What rounding rules must I use for expressing the % DV?
You must express the percentages to the nearest whole percent, except that “Less than 1 %” or “< 1 %” must be used when the amount present is big enough to be listed, but so small that the % DV when rounded to the nearest percent is zero. For example, a product containing 1 gram of total carbohydrate would list the % DV as “Less than 1 %” or “< 1 %.”
So why is any of this important? Because the labeling regulations and FDA guidance say that the Daily Value percentages used on the labels above should be rounded to the nearest whole percent, as they appear to have been. However, the FDA Warning Letter was holding them to the rounding used for conventional foods. And if you are a label reviewer that has been doing it one way or the other, this FDA Warning Letter might have led you to believe you have been doing it incorrectly, or worse, reinforced your incorrect rounding.
Because we review hundreds of labels each year, getting the issue clarified was extremely important. Even though it clearly looked like a mistake on the FDA’s part, there is too much on the line to make that assumption. As such, we sent a letter to the FDA District Office with the question. Although it took a few weeks to get a response, their re-review recognized the error. So if you happened to have had a feeling of déjà vu when you again saw the New Dawn Nutrition, Inc. FDA Warning Letter in late September, it was because it was amended to not include the Daily Value rounding violations and reposted on the FDA’s website.
In the end, all is fixed. Anyone that felt their stomachs drop or lost nights of sleep thinking that all of their Daily Value percent declarations were off can rest knowing that it was all just a minor mistake. There are so many label regulations to keep track of, so it is understandable. At Dietary Supplement Experts, we appreciate the FDA’s swift recognition and correction.
If you are interested, the link to the amended FDA Warning Letter is at http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2015/ucm458141.htm. In addition to the above item of interest, this one is very insightful on cGMP issues, misuse of the FDA logo, proper units of measure, and the findings of the FDA analysis of some of the products will make you wonder how those violations were not in the mainstream news.





 By Curtis Walcker, M.S.
By Curtis Walcker, M.S.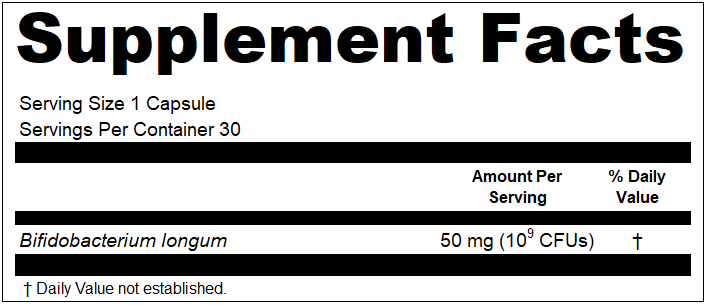


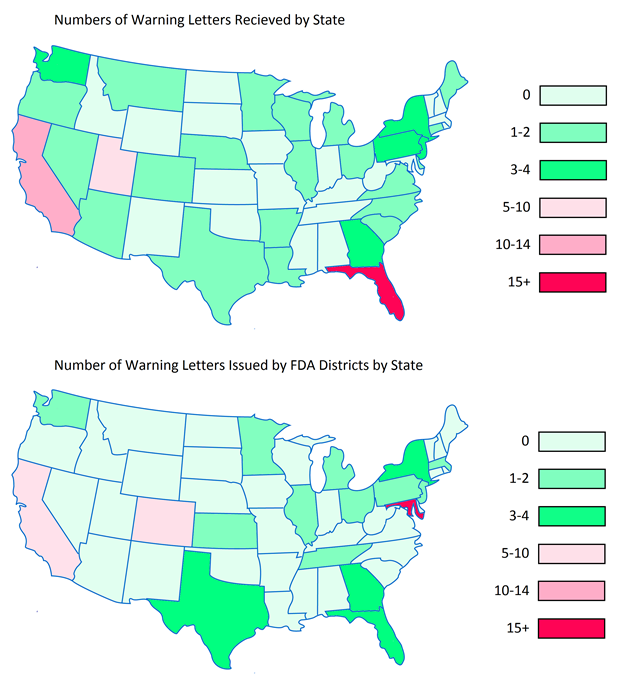
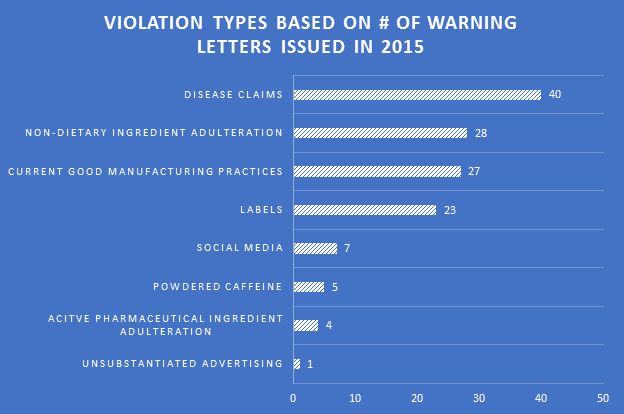
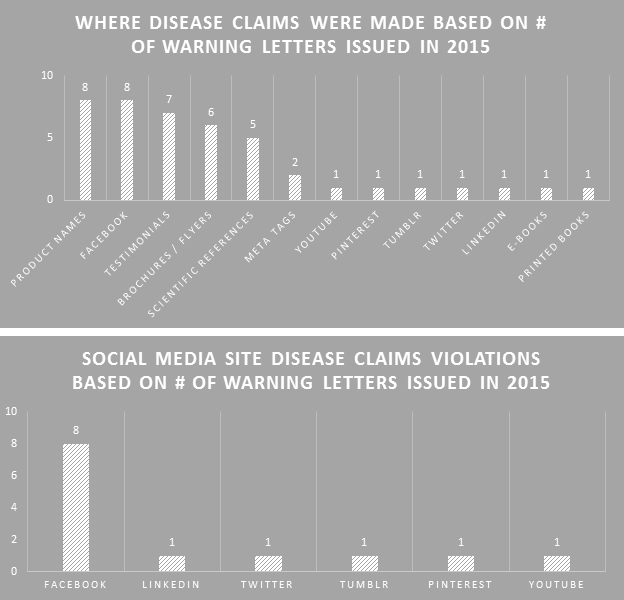
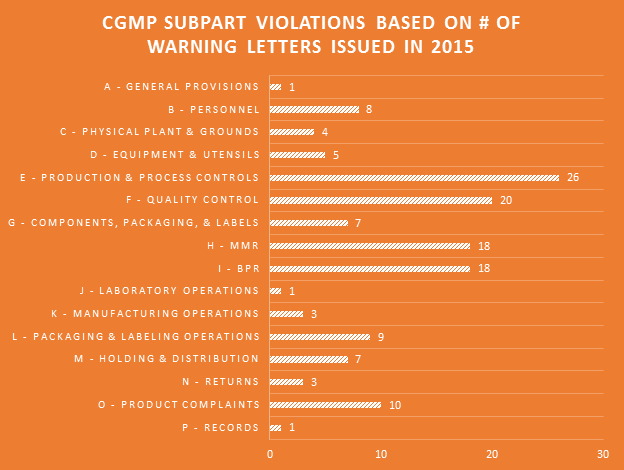
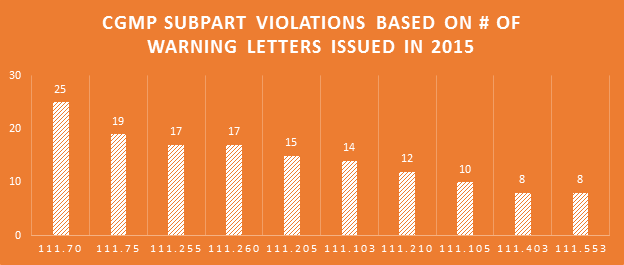
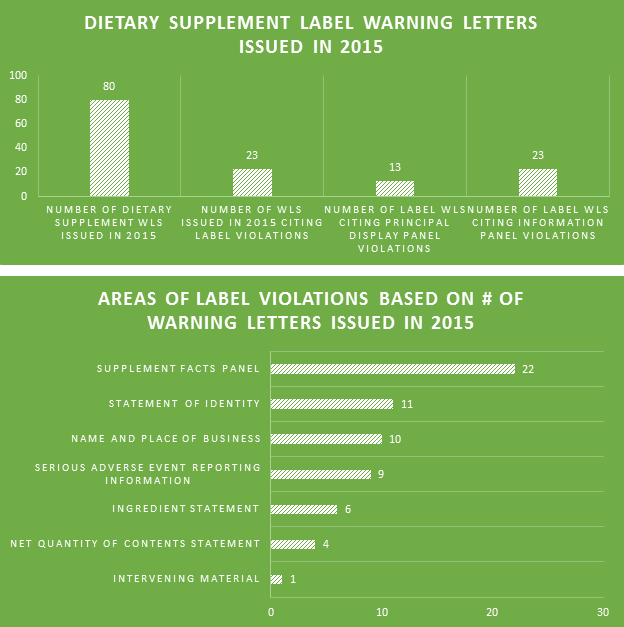
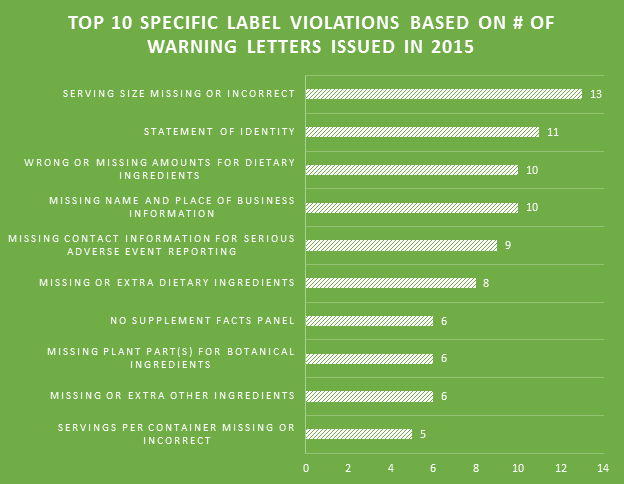

 Many professions require continuing education to keep the professionals in those fields abreast of the trends and evolving research. For us that are involved in dietary supplement regulatory affairs, one of the best unofficial methods of continuing education comes in the form of FDA Warning Letters. A careful reading of these each week not only reminds us of the regulations, but shows us how the FDA is interpreting them, and which issues might be on their radar. In addition, when we are suggesting that our companies or client’s make changes to their manufacturing practices, labels, claims, etc., it can be easier at times to get buy-in when not only the regulations, but also one or more recent FDA Warning Letters can be brought to the discussion table to demonstrate the needs and the associated risks.
Many professions require continuing education to keep the professionals in those fields abreast of the trends and evolving research. For us that are involved in dietary supplement regulatory affairs, one of the best unofficial methods of continuing education comes in the form of FDA Warning Letters. A careful reading of these each week not only reminds us of the regulations, but shows us how the FDA is interpreting them, and which issues might be on their radar. In addition, when we are suggesting that our companies or client’s make changes to their manufacturing practices, labels, claims, etc., it can be easier at times to get buy-in when not only the regulations, but also one or more recent FDA Warning Letters can be brought to the discussion table to demonstrate the needs and the associated risks.

 The FDA posted a new Warning Letter on their site today. The recipient was Yummy Earth Inc., located in New Jersey. It was instantly interesting because this is the second week in a row that the FDA has cited violations for misusing “healthy” nutrient content claims. This new beginning trend started with last week’s letter to KIND, LLC, which has received a considerable amount of attention in the press.
The FDA posted a new Warning Letter on their site today. The recipient was Yummy Earth Inc., located in New Jersey. It was instantly interesting because this is the second week in a row that the FDA has cited violations for misusing “healthy” nutrient content claims. This new beginning trend started with last week’s letter to KIND, LLC, which has received a considerable amount of attention in the press.