By Curtis Walcker, M.S.
January 20, 2015
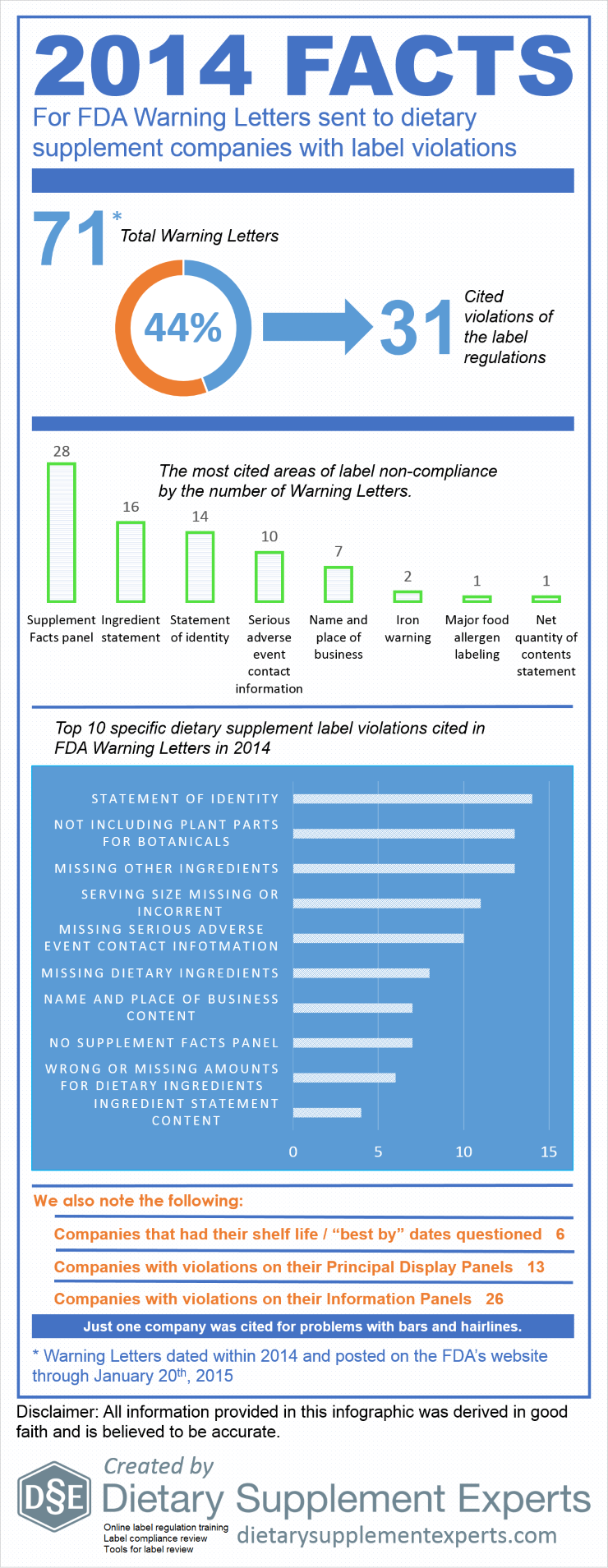
In 2014, 31 of 71 (44%) of FDA Warning Letters sent to dietary supplement companies cited label violations. Compared to 2013, the number of label Warning Letters, as well as the percent were virtually the same (30 letters and 45%, respectively). The Supplement Facts panel, ingredient statement, and statement of identity were the label components holding the most violations. Specifically, the most cited violations (by number of Warning Letters) were improper statements of identity, failing to include the plant parts from which botanical ingredients were derived, and failing to declare ingredients in the ingredient statements, such as the ingredients of capsules.