 By Curtis Walcker, M.S.
By Curtis Walcker, M.S.
September 24, 2018
It’s the age-old label debate – Marketing wants to declare probiotics with CFUs, Regulatory want to declare them in milligrams. Marketing has strong arguments for CFUs – the studies report in CFUs, CFUs make sense for comparing products, competitors use CFUs, and so on. The Regulatory argument is that the regulations require milligrams. So, who is correct? Technically, the Regulatory argument wins here, but as of late, there is more to this story.
Historically, the regulations have required that ingredients such as probiotics 1) be declared with quantitative amounts by weight per serving, and 2) that those amounts be expressed using metric measures in appropriate units, which in most cases for probiotics means milligrams. However, after 20 or so years, the FDA proposed much needed changes to the existing nutrition labels. In the comments submitted, the request was made for the FDA to consider allowing additional units of measure when metric weight is not the most appropriate. Examples given were CFUs for probiotics, and enzyme assay units for digestive enzymes. The FDA responded:
“We decline to permit the use of additional units of measure for dietary ingredients. The comment provided the examples of CFUs for probiotics and enzyme assay units for enzymes; however, the broader change suggested in the comment, by including “other appropriate units of measure,” would allow for the use of units of measure for dietary ingredients other than just probiotics and enzyme assay units.
We recognize that manufacturers are using a number of different units of measure for probiotics, enzymes, and other dietary ingredients. We need to fully evaluate each unit of measure for dietary ingredients to determine if it is appropriate for use on the Supplement Facts label, and if there are any implications to allowing for the use of such units of measure on the label. Because of the complexity of these labeling concerns, we plan to issue information related to this subject at a later date. We have, therefore, finalized § 101.36(b)(2)(ii)(A) without change.”
In early September 2018, the FDA released its Draft Guidance for Industry: Policy Regarding Quantitative Labeling of Dietary Supplements Containing Live Microbials. In this document, the FDA states that they intend to exercise enforcement discretion for companies that choose to declare CFUs in addition to weight for probiotic ingredients if the following seven conditions are met:
- The quantity is first listed in terms of weight;
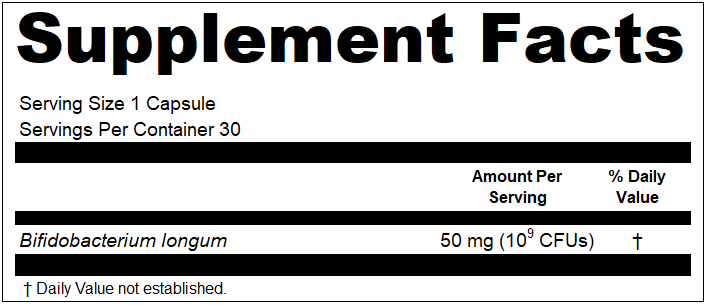
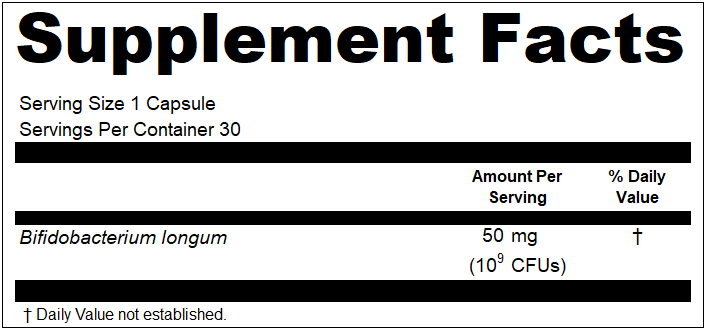
- The declaration of quantity in CFUs is expressed in a manner that is clearly separate and readily distinguishable from the weight, e.g., as a parenthetical or in a subset line;
- The declaration of the quantity in CFUs is formatted in clear terms that can easily be understood by a common reader, e.g., 10 billion or 300* (where the unit that “*” is intended to represent is a typical measurement of CFUs and is clearly indicated elsewhere in the Supplement Facts label);
- The declaration of quantity in CFUs is accurate and not misleading, does not render misleading other aspects of the Supplement Facts label, or other aspects of the product label;
- The declaration of quantity in CFUs measures only live microbial ingredients and does not include inactive, dead, or nonviable organisms;
- Live microbial dietary ingredients in a proprietary blend are listed in descending order of predominance by weight; and
- The product label otherwise complies with all applicable laws and regulations.
Moving forward, it seems that both Regulatory and Marketing groups get what they want for Supplement Facts panels now when it comes to probiotics. The question that remains is how should a compliant label look with both CFUs and milligrams declared? Here is our interpretation for single and dual line declarations: