By Curtis Walcker, M.S.
February 4, 2016
In 2015, the FDA issued 80 Warning Letters (WLs) to dietary supplement (DS) companies. This was a 12.68% increase on the 71 issued in 2014. The increase can in part be attributed to the large number of WLs issued to companies marketing products adulterated with non-dietary ingredients such as beta-methylphenethylamine (BMPEA) and 1,3-dimethylbutylamine (DMBA).
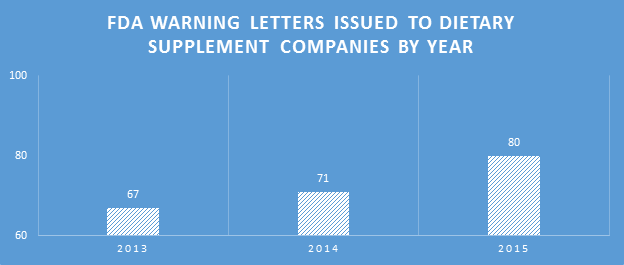
Inspections
Because many of the WLs sent were for the non-dietary ingredient violations, and inspections for those products generally involved review of the labels for ingredients, inspection types categorized as ‘labels’ were significantly higher than other categories in 2015. This did not equate to a proportionally high number of WLs citing label violations.
Inspection types resulting in WLs expanded in 2015. Social media sites have been receiving more scrutiny. The FDA is also looking for claims on online retail sites where companies are marketing DSs, such as Etsy and Amazon.
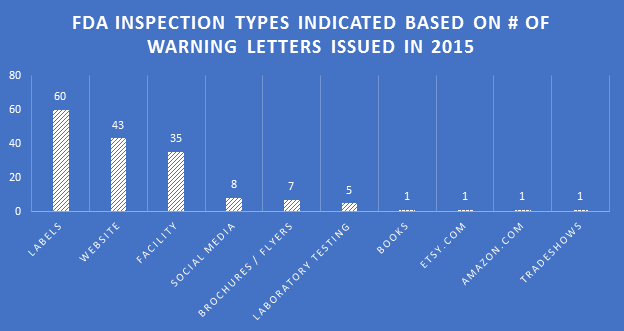
Geographic Locations
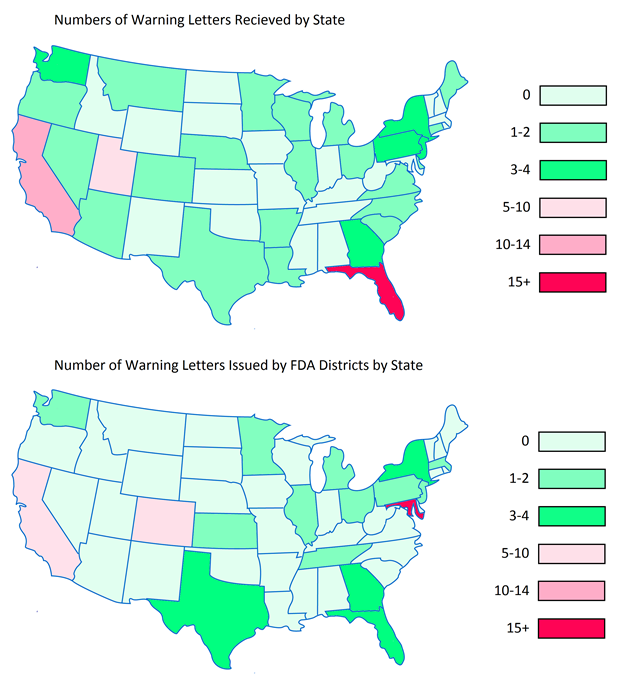
Below are heat maps of the continental Unites States showing the prevalence of WLs received by DS companies by State, as well as which States they were sent from. In 2015, companies in Florida, California, and Utah received the most WLs. The FDA tended to send the most WLs out of the District Offices located in Maryland, California, and Colorado.
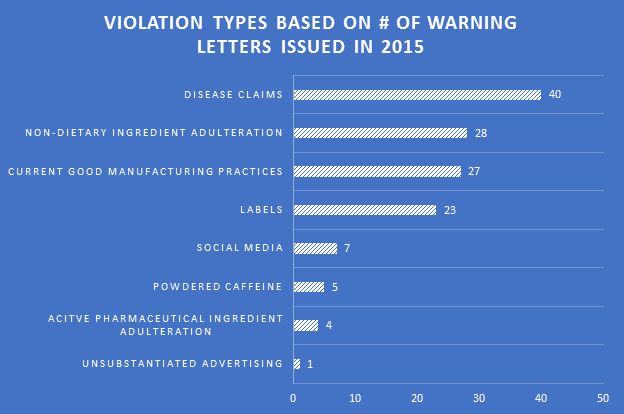
Violations
Disease claims were the most cited violation in WLs for 2015. Non-dietary ingredient adulteration violations were in a distant second place due to some crackdowns on particular ingredients. Violations in current Good Manufacturing Practices (cGMPs) and labels were cited in more than 20 WLs each, indicating continued challenges for companies in both areas.
Disease Claims
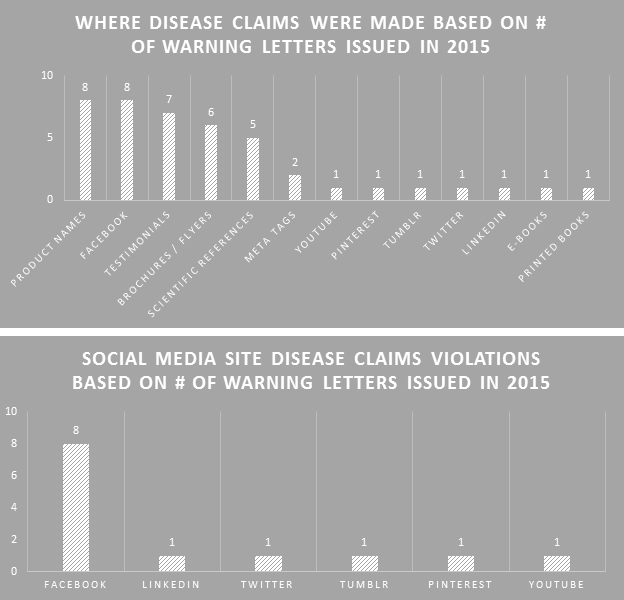
The FDA cited disease claims violations from a variety of places, demonstrating their increasing concern about what companies are saying and where they are saying it. Product names that were disease claims topped the list along with disease claims made on Facebook. New areas in 2015 include e-books, LinkedIn, and Tumblr.
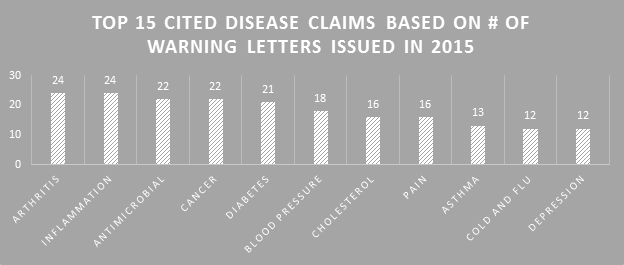
Not much changed in 2015 from previous years in terms of the types of disease claims marketers are illegally using. The top 15 should be no surprise. Combinations of many of these showed up in several of the WLs. The FDA generally only cites disease claims violations in WLs that are clearly explicit.
cGMPs
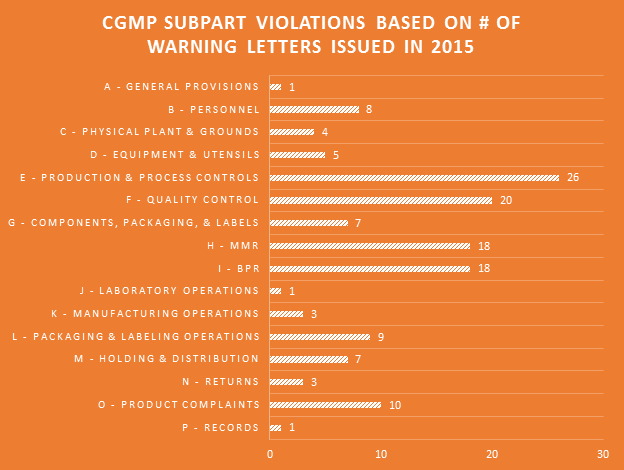
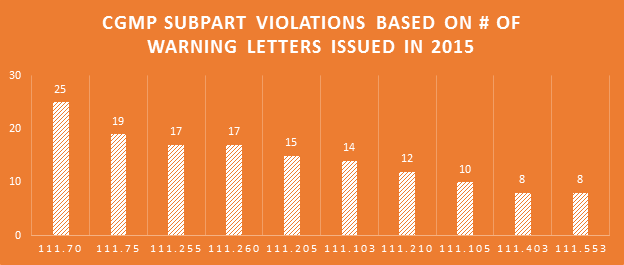
Violations of every Subpart of the cGMP regulations were cited in the 2015 WLs. Production & process controls and quality control Subparts saw the most violations. Companies failing to have adequate master manufacturing records (MMRs) and batch production records (BPRs) continues to be a major problem.
Labels
Of the 80 DS WLs issued in 2015, nearly 30% of them cited violations for product labels. This number is likely deceivingly low because so many of the non-dietary ingredient WLs seemingly failed to also cite label violations for the offending products. All 23 WLs citing label violations highlighted errors within the information panels; only 13 involved errors within the principal display panels.
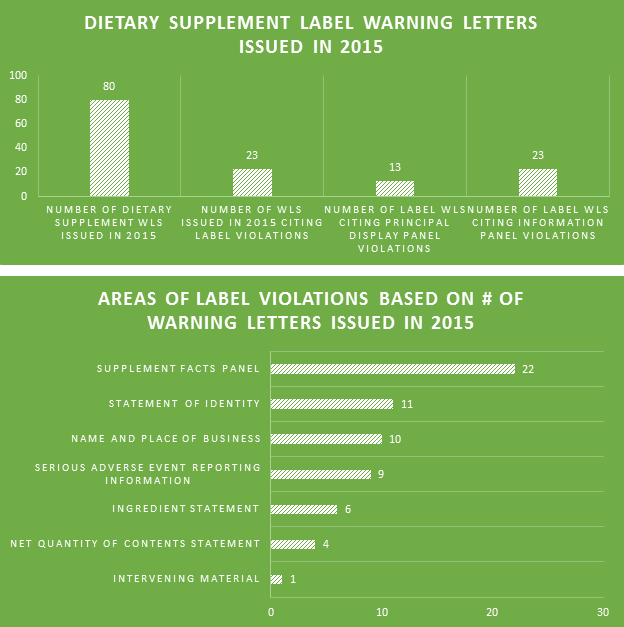
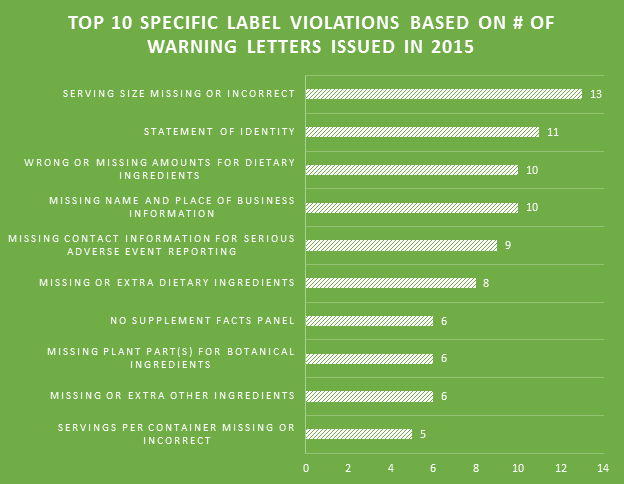
One of the biggest label violations of 2015 involved matching the label’s recommended usage of the product to the declared Serving Size. The majority of the top 10 violations were made within Supplement Facts panels. Failing to include proper contact information for the reporting of serious adverse events associated with product use is a prevalent problem among labels.
Note: All information in this article was derived in good faith and is believed to be accurate. Data was collected from all 2015 FDA Warning Letters issued to dietary supplement companies posted on the FDA’s website as of February 3, 2016.

