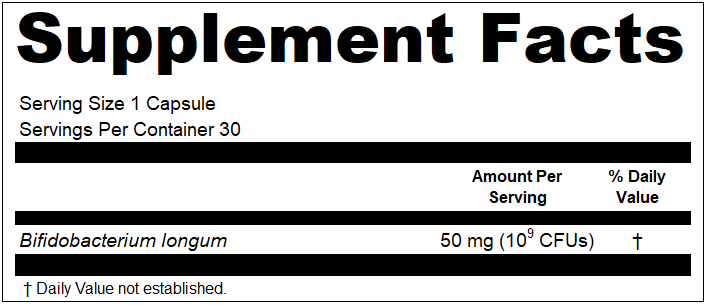
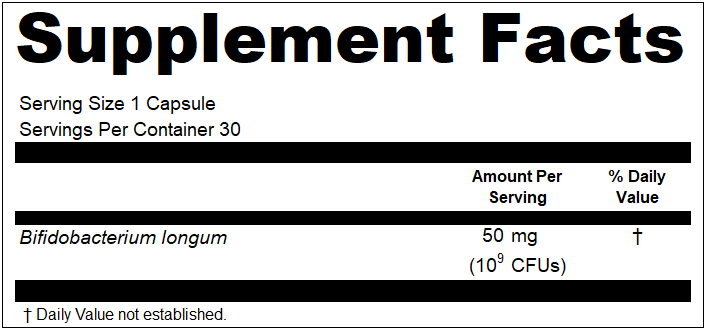
Over the years, we have been approached multiple times about artificial intelligence programs for reviewing and correcting dietary supplement copy for FDA compliance. We have been suggested by clients to create it. We have been asked by tech groups to co-create it. We have been pitched it by those that have attempted it. And while the thought of it can be a little exciting, that excitement quickly fades once you start mentally pressure testing the concept, and sorting through the countless ways it would be likely to fail you, and possibly increase risks while simultaneously “mitigating” risks.
Before you go off-loading your regulatory compliance onto a computer program, we have compiled a list of things you should consider. And while our rough list topped 20, we will give you our top 9.
- Content is King, but context is Queen. While a program might be able to scan for words and phrases in isolation, it could be difficult to recognize two things that might be okay in isolation, but create an impermissible claim when paired together.
Example: Claim says “Supports joint comfort” with an adjacent image of an arthritic hand with red enhancements to portray pain and inflammation. The claim might be okay on its own, but quickly becomes a high-risk disease claim in the context of the image. - Finding implied disease claims. You often get out of something what you put into it. As could be the case with programs looking specifically for human-inputted disease terms. An implied disease claim can be just as risky as an explicit one.
Example: If the program screens for the terms “diabetes” and “hyperglycemia”, and overlooks the implied disease claims “our product can reduce your need to take insulin” or “improves fasting glucose and HbA1c”. - Overlooking images that create disease claims. Many brands do not understand that images, vignettes, and symbols can constitute disease claims. It might be challenging enough loading a program up with words and phrases
,but trusting it to catch an image of an abnormal tissue or organ is even more difficult.
Example: Your product is for respiratory health and all copy is compliant. However, an image is used of a woman sneezing with pollens floating in the air. Would that be nuanced enough to be caught? - Catching disease terms at times where they are okay. Just because a disease term is used, does not automatically mean it is non-compliant and in need of editing or removal.
Example: You feature a customer testimonial that mentions disease, but is not making any disease claims about the product. “After COVID lockdowns, we finally returned to in-person classes at the university. Product X has helped me focus on my studies.” - Not accounting for claims substantiation. Making disease claims may land you in some trouble, but making unsubstantiated claims may drain your bank. Claims must align with the support behind them. AI is not going to know if your claims are truthful, not misleading, and adequately substantiated.
Example: Your product features the latest trending ingredient. Some of your competitors are claiming this ingredient helps with weight loss, improves sleep quality, reduces stress levels, increases energy, and so on. Nothing necessarily claiming to treat or prevent diseases. But your only substantiation is “well Brand A and Brand B say it, so it must be true.” Your risk persists. - Text in images. On some advertising and distribution platforms, programs are already being used to search for higher-risk terms in copy. It did not take long for marketers to realize that they could cheat some of these systems by incorporating disease claims into images so the text cannot be found. Will programs recognize text in images (not to mention videos)?
Example: All your copy is compliant, but you make a disease claim hidden in one of your images. - Overlooking scientific references. While having scientific references on hand to support claims is critical, it might not always be the best idea to publish their citations in marketing copy. Especially if those references involve disease outcomes, and imply that a product will also impact disease. Brands have often attempted to mitigate risks in this area by omitting parts of citations such as the title, as to remove some disease words from a page. It often helps, but not always.
Example: You have a study to support a specific claim: Zhao JV, et al. Effect of Berberine on Cardiovascular Disease Risk Factors: A Mechanistic Randomized Controlled Trial. Nutrients. 2021 Jul 26;13(8):2550. You opt to remove the title to take “cardiovascular disease” off the page and publish as: Zhao JV, et al. Nutrients. 2021 Jul 26;13(8):2550. When a program might have found the offending terms before, it would likely miss this “sanitized” version. - Wordsmithing and editing can be difficult. For most disease claims, there can be an alternate phrasing applied to make them structure/function claims while keeping similar contextual meaning, still aligning with the supporting science, and satisfying both compliance and marketing folks. Sometimes these edits are easy, but other times it involves a lot of debate and creativity. It would make sense that programs could suggest changes or give guidance on flagged claims, but without full consideration, the correction might just create a whole other problem.
Example: A program flags a claim that an ingredient of a product was shown to reduce blood pressure in a major clinical trial,and provides guidance that blood pressure claims should be written to the effect of “maintaining already healthy blood pressure in healthy individuals”. The claim gets corrected in this way, but no longer aligns with the study that might have involved a significantly hypertensive population that saw modest improvements with a treatment. - Missing that human touch. Regulatory work is black and white on its face, but the grey reality involves a lot of context and nuance and consideration of individual brands’ risk tolerances. What might be a good strategy today, can change in an instant following changing laws or regulations, new guidance and policies, enforcement trends, industry trends, emerging health conditions, and so on. How often will your program update to account for the changes? And factoring back in the human touch with technologies will still be a challenge with regards to training, interpretations, user/employee turnover, marketers refusing to accept regulatory changes, and many of the normal challenges we already deal with daily without adding a computer program to the mix.
Admittedly, creating a list like this does stir some creativity, and ideas on how to overcome these challenges. However, it is still very difficult to trust that issues like these and a myriad of others will be solved in the foreseeable future. But for now, we are not suggesting that clients use artificial intelligence to write marketing copy, and we are not at all suggesting that clients trust artificial intelligence to ensure their copy is compliant.







 If you are a frequent reviewer of dietary supplement labels, you have probably found that your speed and efficiency increase when you have your “tools” readily accessible. All the way back in 2014, we published the
If you are a frequent reviewer of dietary supplement labels, you have probably found that your speed and efficiency increase when you have your “tools” readily accessible. All the way back in 2014, we published the 
 By Curtis Walcker, M.S.
By Curtis Walcker, M.S.